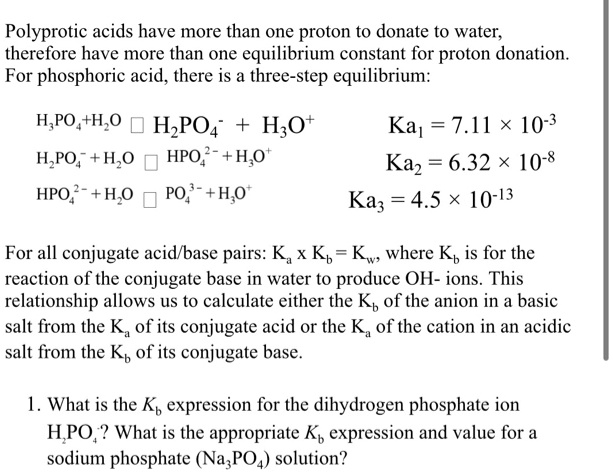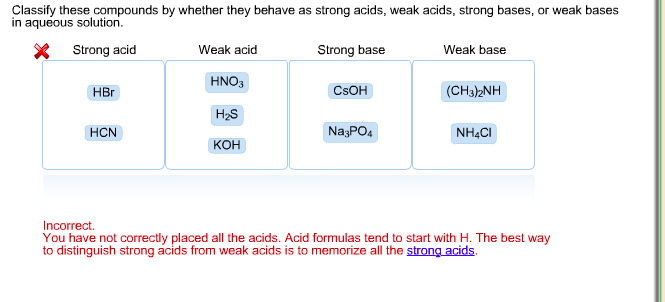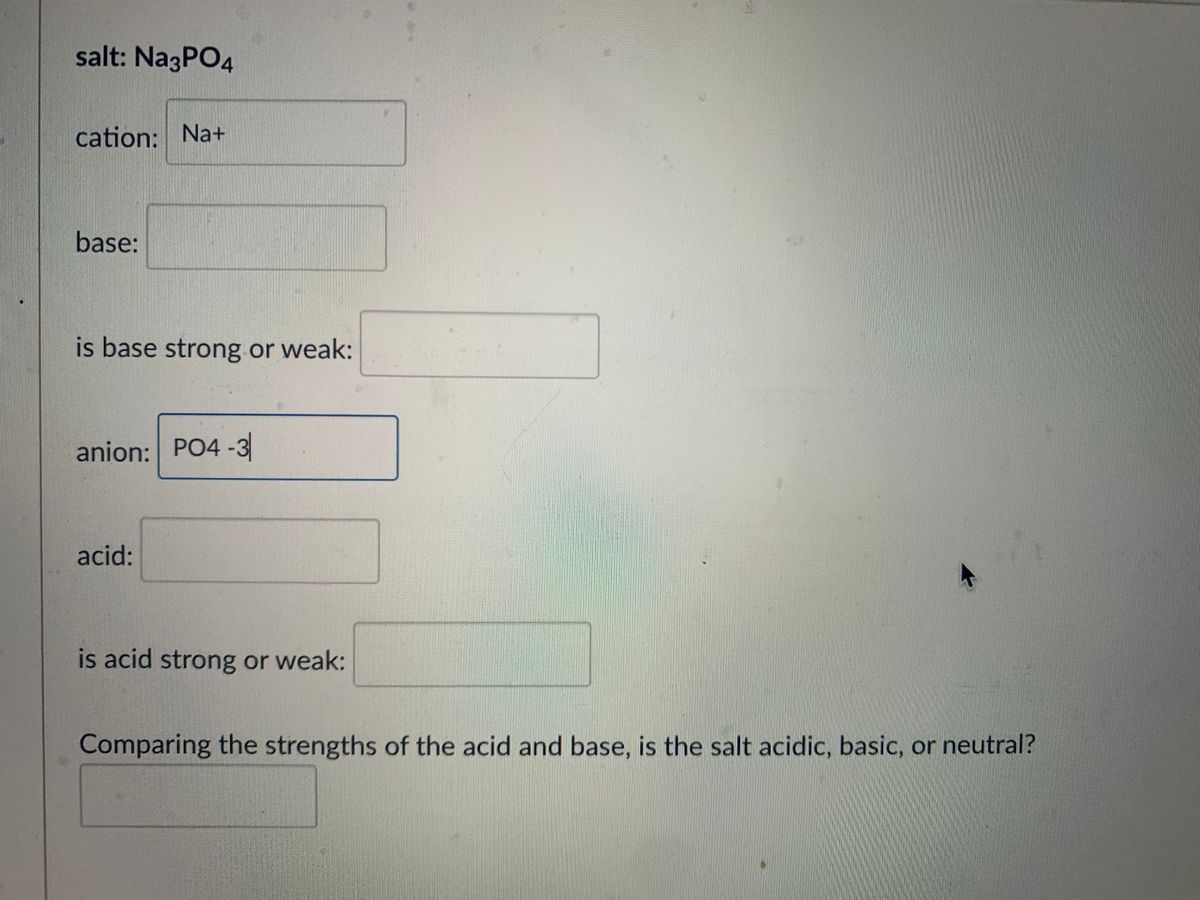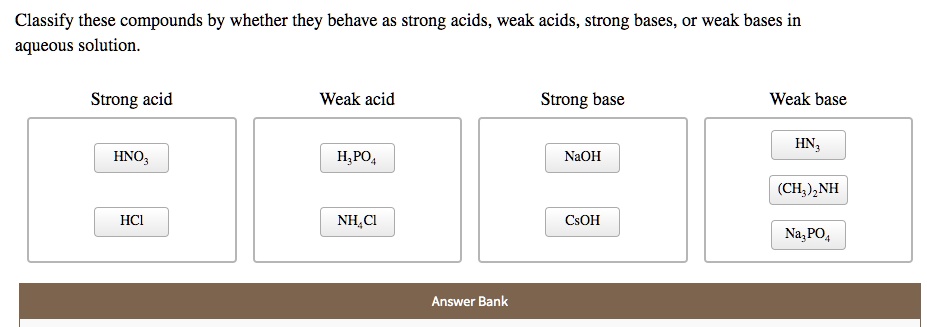
SOLVED: Classify these compounds by whether they behave as strong acids, weak acids, strong bases or weak bases in aqueous solution. Strong acid Weak acid Strong base Weak base IN, IINO; I,PO4

SOLVED: The household cleaner TSP contains sodium phosphate as the active ingredient: In solution the phosphate ion reacts with water as shown in the following equation. PO4(aq) H,O(aq) HPO4aq) + OH(aq) (a)
![Expert Answer] classify the following salts as neutral , acidic or basic. also,write their name. a) - Brainly.in Expert Answer] classify the following salts as neutral , acidic or basic. also,write their name. a) - Brainly.in](https://hi-static.z-dn.net/files/d3d/3e43c78b8d42fd1bf93f75db19f306ce.jpg)
Expert Answer] classify the following salts as neutral , acidic or basic. also,write their name. a) - Brainly.in

SOLVED: Question 3 (4 points) Listen The following equation indicates that Na3PO4 is: HzO Na3 PO4(s) 3Na + (aq) PO43- (aq) a weak electrolyte strong electrolyte non-electrolyte an acid a base